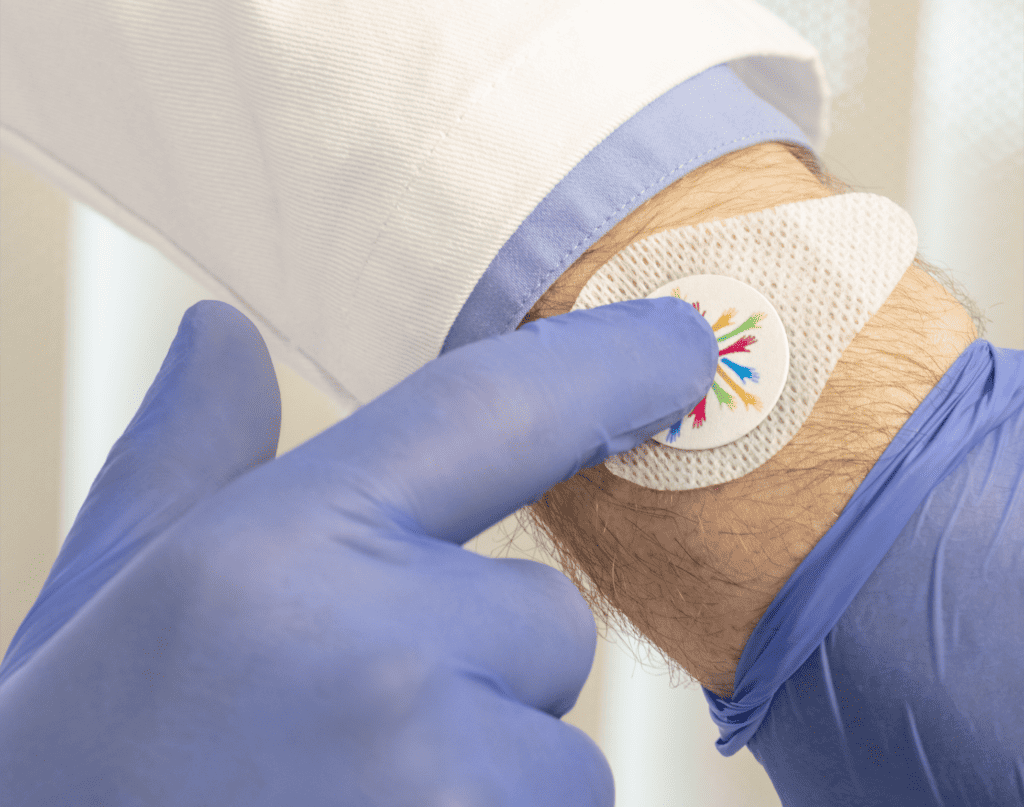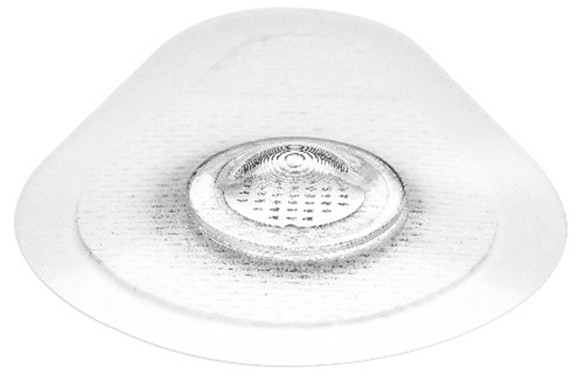
VaxiPatch™ Microneedle Array
The VaxiPatch™ Is Engineered For Precise Dosing
Verndari’s proprietary microneedle design was developed to ensure precise vaccine dose dispensing. Verndari’s VaxiPatch™ coated with vaccine is integrated into single-use applicator, ready-for-vaccination. The product is designed to be effective and easy-to-use, while meeting industrial-scale manufacturing and commercial logistics requirements.The VaxiPatch™ was designed for high-speed, high-volume, automated manufacturing using low-cost and readily available materials including medical grade stainless steel. Verndari is developing the VaxiPatch™ to be manufactured using GMP-compliant, automated, aseptic fill/finish systems.